Details of the Drug
General Information of Drug (ID: DMRUIX3)
| Drug Name |
GP515
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1H-Pyrazolo(3,4-d)pyrimidin-4-amine, 1-(5-amino-5-deoxy-beta-D-ribofuranosyl)-3-bromo-; GP 1-515; CHEMBL356141; 144928-48-3; GP-515; Adenosine kinase inhibitor GP515; GP-1-515; SCHEMBL2053010; AC1L31E2; DTXSID30162847; BDBM50134744; 1-(5-Amino-5-deoxy-beta-D-ribofuranosyl)-3-bromo-1H-pyrazolo(3,4-d)pyrimidin-4-amine; 4-Amino-1-(5-amino-5-deoxy-1-beta-D-ribofuranosyl)-3-bromopyrazolo(3,4-d)pyrimidine; (2R,3R,4S,5R)-2-(4-amino-3-bromopyrazolo[3,4-d]pyrimidin-1-yl)-5-(aminomethyl)oxolane-3,4-diol; 19-nor-10-azasteroid skeleton
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
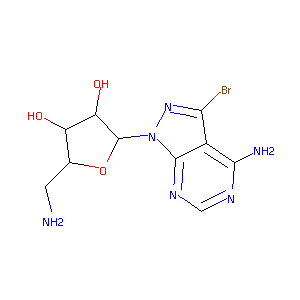 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 345.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
