Details of the Drug
General Information of Drug (ID: DMRV89X)
| Drug Name |
Cl-amidine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cl-amidine; CHEMBL1962361; N-[(1S)-1-(Aminocarbonyl)-4-[(2-chloro-1-iminoethyl)amino]butyl]-benzamide; 913723-61-2; CHEMBL1910972; GTPL8685; US8969333, CI-amidine; SCHEMBL1979577; BDBM144279; ZINC71746281; BDBM50355657; 4373AJ; NE62957; HY-100574; CS-0019714; FT-0700077; (2S)-5-(2-chloroethanimidamido)-2-(phenylformamido)pentanamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
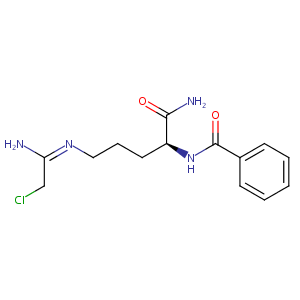 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 310.78 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
