Details of the Drug
General Information of Drug (ID: DMRWSKX)
| Drug Name |
Afegostat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Isofagomine; (3R,4R,5R)-5-(HYDROXYMETHYL)PIPERIDINE-3,4-DIOL; 169105-89-9; iso-Fagomine; D-Isofagomine; UNII-G23AP190YS; 5-HYDROXYMETHYL-3,4-DIHYDROXYPIPERIDINE; CHEMBL206468; G23AP190YS; IFM; 5-Hydroxymethyl-3,4-piperidinediol; (3R,4R,5R)-5-(HYDROXYMETHYL)PIPERIDINE-3,4-DIOL HYDROCHLORIDE; Afegostat [USAN:INN]; 1oif; Afegostat (USAN/INN); SCHEMBL581577; GTPL7410; 3,4-piperidinediol, 5-(hydroxymethyl)-, (3R,4R,5R)-; DTXSID20168651; 5-Hydroxymethylpiperidine-3,4-diol; ZINC3813668; BDBM50182801; AKOS006348872; DB04545; HY-14829; CS-0003581; D09576; Q21396373; (3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8; Afegostat; isofagomine; (3R,4R,5R)-5-(HYDROXYMETHYL)PIPERIDINE-3,4-DIOL
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
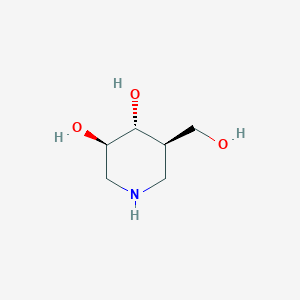 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 147.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
