Details of the Drug
General Information of Drug (ID: DMRYM03)
| Drug Name |
HMS5552
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dorzagliatin; UNII-X59W6980E8; X59W6980E8; 1191995-00-2; Sinogliatin; RO5305552; Dorzagliatin [INN]; Dorzagliatin [USAN:INN]; SCHEMBL1079619; HMUMWSORCUWQJO-QAPCUYQASA-N; HMS-5552; AKOS030527745; RO-5305552; (S)-2-(4-(2-chlorophenoxy)-2-oxo-2,5; (S)-2-(4-(2-chlorophenoxy)-2-oxo-2,5-dihydro-1H-pyrrol-1-yl)-N-(1-((R; (S)-2-[4-(2-chloro-phenoxy)-2-oxo-2,5-dihydro-pyrrol-1-yl]-4-methyl-pentanoic acid [1-((R)-2,3-dihydroxy-propyl)-1H-pyrazol-3-yl]-amide; (2S)-2-(4-(2-chlorophenoxy)-2-oxo-2,5-dihydro-1H-pyrrol-1-yl)-N-(1
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Structure |
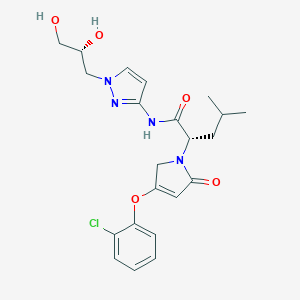 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 462.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Type-2 diabetes | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A11 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
