Details of the Drug
General Information of Drug (ID: DMS0GA6)
| Drug Name |
AMY-101
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-4Z4DFR9BX7; 4Z4DFR9BX7; 1427001-89-5 (free base); Compstatin 40; Compstatin analog peptide CP40; CHEMBL4297260; 1427001-89-5; L-Isoleucinamide, D-tyrosyl-L-isoleucyl-L-cysteinyl-L-valyl-1-methyl-L-tryptophyl-L-glutaminyl-L-alpha-aspartyl-L-tryptophyl-N-methylglycyl-L-alanyl-L-histidyl-L-arginyl-L-cysteinyl-N2-methyl-, cyclic (3->13)-disulfide; S3,S13-Cyclo(D-tyrolsyl-L-isoleucyl-L-cysteinyl-L-valyl-1-methyl-L-tryptophyl-L-glutaminyl-L-aspartyl-L-tryptophyl-N-methyl-L-glycyl-L-alanyl-L-histidyl-L-arginyl-L-cysteinyl-N-methyl-L-isoleucinamide)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Peptide
|
||||||||||||||||||||||
| Structure |
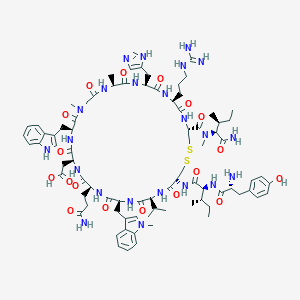 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1789.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 30 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 21 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 23 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
