Details of the Drug
General Information of Drug (ID: DMS1DMA)
| Drug Name |
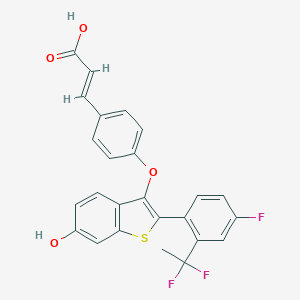
LSZ102
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
SJXNPGGVGZXKKI-NYYWCZLTSA-N; LSZ-102; 2135600-76-7; (E)-3-(4-((2-(2-(1,1-difluoroethyl)-4-fluorophenyl)-6-hydroxybenzo[b]thiophen-3-yl)oxy)phenyl)acrylic acid; SCHEMBL17334098; BDBM269484; EX-A1874; BCP29222; HY-111486; CS-0042193; US10058534, 139; (E)-3-(4-((2-(2-(1,1-difluoroethyl)-4-fluorophenyl)-6-hydroxybenzo [b]thiophen-3-yl)oxy)phenyl)acrylic acid; C6V; (2E)-3-[4-({2-[2-(1,1-difluoroethyl)-4-fluorophenyl]-6-hydroxy-1-benzothiophen-3-yl}oxy)phenyl]prop-2-enoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
