Details of the Drug
General Information of Drug (ID: DMS80DF)
| Drug Name |
ALDISIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
72908-87-3; Aldisin; Aldisine; 6,7-dihydropyrrolo[2,3-c]azepine-4,8(1H,5H)-dione; 6,7-Dihydro-1H,5H-pyrrolo[2,3-c]azepine-4,8-dione; CHEMBL357047; AK134979; Pyrrolo[2,3-c]azepine-4,8(1H,5H)-dione, 6,7-dihydro-; Pyrrolo(2,3-c)azepine-4,8(1H,5H)-dione, 6,7-dihydro-; AC1MJ57G; SCHEMBL7844916; 6,7-dihydro-Pyrrolo[2,3-c]azepine-4,8(1H,5H)-dione; CTK5D7039; KS-00000FCK; DTXSID40223211; Pyrrolo[2,3-c]azepine-4,8(1H,5H)-dione,6,7-dihydro-; AAPGLCCSVSGLFH-UHFFFAOYSA-N; MolPort-000-003-213; ZINC13310235; BDBM50108777; FCH858612
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
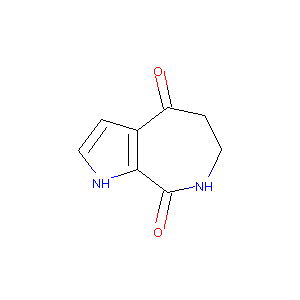 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 164.16 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
