Details of the Drug
General Information of Drug (ID: DMSA27Z)
| Drug Name |
MK-6186
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
MK-6186; UNII-K3978U665M; 1034474-19-5; K3978U665M; MK 6186; SCHEMBL2878768; CHEMBL1939499; FZBAOOQVQXATRL-UHFFFAOYSA-N; ZINC73240561; DB12999; DA-16093; 3-(1-((1H-pyrazolo[3,4-b]pyridin-3-yl)methyl)-5-chloro-1H-indazol-4-yloxy)-5-chlorobenzonitrile; FT-0726721; 3-((1-((2H-pyrazolo[3,4-b]pyridin-3-yl)methyl)-5-chloro-1H-indazol-4-yl)oxy)-5-chlorobenzonitrile; 3-chloro-5-{[5-chloro-1-(1H-pyrazolo[3,4- b]pyridin-3-ylmethyl)-1H-indazol-4-yl]oxy}benzonitrile
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
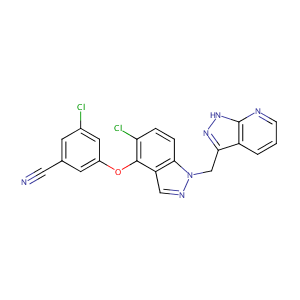 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 435.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
