Details of the Drug
General Information of Drug (ID: DMSJLX0)
| Drug Name |
UBP-302
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UBP 302; (S)-1-(2-AMINO-2-CARBOXYETHYL)-3-(2-CARBOXYBENZYL)PYRIMIDINE-2,4-DIONE; UBP302; CHEMBL372797; 745055-91-8; UBC; Tocris-2079; AC1O4WKY; SCHEMBL2265710; GTPL4333; DTXSID80423571; MolPort-003-983-705; 2f35; ZINC3996041; BDBM50178136; BN0533; AKOS024456932; NCGC00025352-01; UBP302, > B6942; SR-01000597533; SR-01000597533-1; (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl)-pyrimidine-2,4-dione; 2-[[3-[(2S)-2-amino-3-hydroxy-3-oxopropyl]-2,6-dioxopyrimidin-1-yl]methyl]benzoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
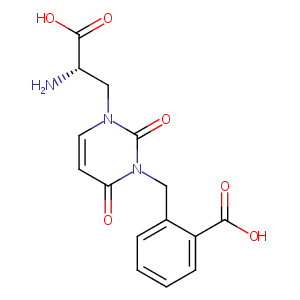 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 333.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
