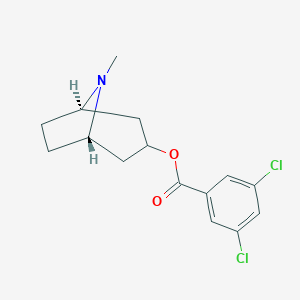
| Synonyms |
3-Tropanyl-3,5-dichlorobenzoate; MDL 72222; MDL-72222; C15H17Cl2NO2; CHEMBL376379; 40796-97-2; Tropyl 3,5-dichlorobenzoate; 8-Methyl-8-azabicyclo[3.2.1]oct-3-yl 3,5-dichlorobenzoate; Bemesetron [USAN:INN]; Bemesetronum [INN-Latin]; 3,5-Dichloro-benzoic acid 8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl ester; SR-01000075587; Tropanyl 3,5-dichlorobenzoate; 1alphaH,5alphaH-Tropan-3alpha-yl 3,5-dichlorobenzoate; endo-8-Methyl-8-azabicyclo(3.2.1)oct-3-yl 3,5-dichlorobenzoate; (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 3,5-dichlorobenzo
|
| Chemical Identifiers |
- Formula
- C15H17Cl2NO2
- IUPAC Name
[(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 3,5-dichlorobenzoate - Canonical SMILES
-
CN1[C@@H]2CC[C@H]1CC(C2)OC(=O)C3=CC(=CC(=C3)Cl)Cl
- InChI
-
InChI=1S/C15H17Cl2NO2/c1-18-12-2-3-13(18)8-14(7-12)20-15(19)9-4-10(16)6-11(17)5-9/h4-6,12-14H,2-3,7-8H2,1H3/t12-,13+,14?
- InChIKey
-
MNJNPLVXBISNSX-PBWFPOADSA-N
|