Details of the Drug
General Information of Drug (ID: DMSTZ14)
| Drug Name |
A-395
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL4104741; (3R,4S)-1-(7-fluoro-2,3-dihydro-1H-inden-1-yl)-N,N-dimethyl-4-(4-(4-(methylsulfonyl)piperazin-1-yl)phenyl)pyrrolidin-3-amine; GTPL9525; SCHEMBL19549392; BDBM223987; BDBM50241662; A-395 hydrochloride, >=98% (HPLC); HY-101512; CS-0021615; J3.609.234C; A-395 (5); A392089148-72-9; (3R,4S)-1-(7-fluoro-2,3-dihydro-1H-inden-1-yl)-N,N-dimethyl-4-[4-(4-methylsulfonylpiperazin-1-yl)phenyl]pyrrolidin-3-amine; 2089148-71-8; rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H-inden-1-yl)-N,N-dimethyl-4-(4-(4-(methylsulfonyl)piperazin-1-yl)phenyl)pyrrolidin-3-amine (4); rel-(3R,4S)-1-(7-Fluoro-2,3-dihydro-1H-inden-1-yl)-N,N-dimethyl-4-(4-(4-(methylsulfonyl)piperazin-1-yl)phenyl)pyrrolidin-3-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
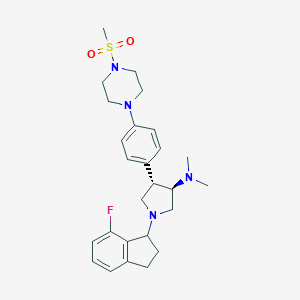 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 486.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
