Details of the Drug
General Information of Drug (ID: DMTBFE4)
| Drug Name |
LY2090314
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
603288-22-8; LY-2090314; UNII-822M3GYM67; Kinome_3681; LY 2090314; CHEMBL362558; 822M3GYM67; 3-(9-Fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetrahydro-[1,4]diazepino[6,7,1-hi]indol-7-yl)-4-(imidazo[1,2-a]pyridin-3-yl)-1H-pyrrole-2,5-dione; SCHEMBL633455; GTPL7958; DTXSID90209085; MolPort-035-944-332; EX-A2214; ZINC3817327; BCP07855; s7063; BDBM50150699; AKOS032950045; AKOS026750195; CS-1633; DB11913; SB16558; NCGC00378942-05; NCGC00378942-02; BC600682; QC-11735; HY-16294; KB-78238; FT-0698670; LY2090314, >
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
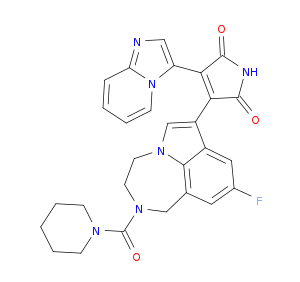 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 512.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
