Details of the Drug
General Information of Drug (ID: DMTV7XH)
| Drug Name |
SPN-812
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Viloxazine hydrochloride; 35604-67-2; 2-((2-Ethoxyphenoxy)methyl)morpholine hydrochloride; Emovit; 2-[(2-ethoxyphenoxy)methyl]morpholine hydrochloride; ICI 58,834; Viloxazine hydrochloride [USAN]; 2-((2-ethoxyphenoxy)methyl)morpholinehydrochloride; 2-[(2-ethoxyphenoxy)methyl]morpholine;hydrochloride; Morpholine, 2-[(2-ethoxyphenoxy)methyl]-, hydrochloride; Vicilan; Vivalan; 2-[(2-ethoxy phenoxy)methyl]morpholine hydrochloride; Morpholine, 2-((2-ethoxyphenoxy)methyl)-, hydrochloride; Viloxazine hydrochloride (USAN); Viloxazine HCl; CCRIS 1915; Viloxacina clorhidrato; ICI-58834; Viloxacina clorhidrato [Spanish]; EINECS 252-638-6; Prestwick_734; 2-((o-Ethoxyphenoxy)methyl)morpholine hydrochloride; Vivalan (TN); 2-(2-Ethoxyphenoxymethyl)tetrahydro-1,4-oxazine hydrochloride; 2-(2-Ethoxyphenoxymethyl)-2,3,5,6-tetrahydro-1,4-oxazine hydrochloride; rac Viloxazine Hydrochloride; DSSTox_CID_31511; DSSTox_RID_97396; DSSTox_GSID_57722; SCHEMBL300741; CHEMBL2106483; DTXSID8057722; Tox21_113955; 1478AE; AKOS015847050; CCG-220828; NCGC00262964-01; CAS-35604-67-2; A6217; FT-0675815; FT-0675816; FT-0675817; FT-0675818; FT-0675819; D02572; Z-1958; 2-(o-ethoxyphenoxymethyl)morpholine hydrochloride; 604E672; SR-01000872636; SR-01000872636-1; W-110863; 2-(2-Ethoxy-phenoxymethyl)-morpholine; hydrochloride; 2-[(2-Ethoxyphenoxy)-methyl]morpholine hydrochloride; Q27285797; 2-(2-ethoxy-phenoxymethyl)-tetra-hydro-1,4-oxazine hydrochloride
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
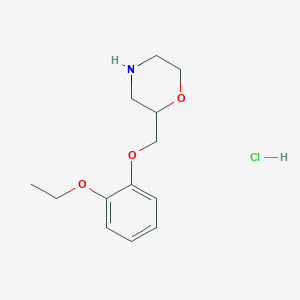 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 273.75 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Attention deficit hyperactivity disorder | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A05.Z | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
