| Drug Name |
U-92032
|
| Synonyms |
U-92032; U 92032; CHEMBL327057; 142223-92-5; AC1L30XY; SCHEMBL195024; DTXSID70161975; BDBM50091592; u92032; 7-((4-(Bis(4-fluorophenyl)methyl)-1-piperazinyl)methyl)-2-((2-hydroxyethyl)amino)-4-(1-methylethylethyl)-2,4,6-cycloheptatrien-1-one; 7-{4-[Bis-(4-fluoro-phenyl)-methyl]-piperazin-1-ylmethyl}-2-(2-hydroxy-ethylamino)-4-isopropyl-cyclohepta-2,4,6-trienone(U-92032); 2,4,6-Cycloheptatrien-1-one, 7-((4-(bis(4-fluorophenyl)methyl)-1-piperazinyl)methyl)-2-((2-hydroxyethyl)amino)-4-(1-methylethyl)-
|
| Drug Type |
Small molecular drug
|
| Structure |
|
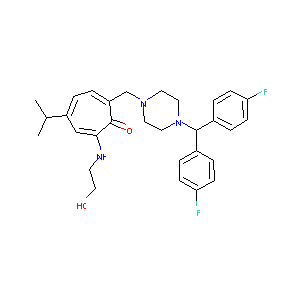 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight (mw) |
507.6 |
|
| Logarithm of the Partition Coefficient (xlogp) |
4.8 |
| Rotatable Bond Count (rotbonds) |
9 |
| Hydrogen Bond Donor Count (hbonddonor) |
2 |
| Hydrogen Bond Acceptor Count (hbondacc) |
7 |
| Chemical Identifiers |
- Formula
- C30H35F2N3O2
- IUPAC Name
7-[[4-[bis(4-fluorophenyl)methyl]piperazin-1-yl]methyl]-2-(2-hydroxyethylamino)-4-propan-2-ylcyclohepta-2,4,6-trien-1-one - Canonical SMILES
-
CC(C)C1=CC=C(C(=O)C(=C1)NCCO)CN2CCN(CC2)C(C3=CC=C(C=C3)F)C4=CC=C(C=C4)F
- InChI
-
InChI=1S/C30H35F2N3O2/c1-21(2)24-3-4-25(30(37)28(19-24)33-13-18-36)20-34-14-16-35(17-15-34)29(22-5-9-26(31)10-6-22)23-7-11-27(32)12-8-23/h3-12,19,21,29,36H,13-18,20H2,1-2H3,(H,33,37)
- InChIKey
-
UVPCKMJVJLKETQ-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 132417
- CAS Number
-
- TTD ID
- D0OW2O
|
|
|
|
|
|
|
|

