Details of the Drug
General Information of Drug (ID: DMU26OE)
| Drug Name |
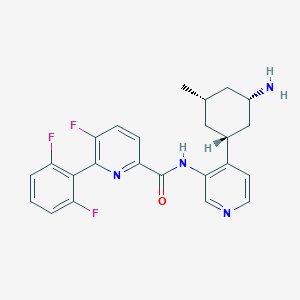
PIM447
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
VRQXRVAKPDCRCI-ZNMIVQPWSA-N; PIM-447; 1210608-43-7; PIM447 (LGH447); N-{4-[(1r,3s,5s)-3-Amino-5-Methylcyclohexyl]pyridin-3-Yl}-6-(2,6-Difluorophenyl)-5-Fluoropyridine-2-Carboxamide; N-[4-[(1R,3S,5S)-3-amino-5-methylcyclohexyl]pyridin-3-yl]-6-(2,6-difluorophenyl)-5-fluoropyridine-2-carboxamide; UNII-9TG5O4V25H; SCHEMBL3215332; GTPL9790; 9TG5O4V25H; CHEMBL3651966; PIM 447 [WHO-DD]; BDBM106870; ZINC116907270; AKOS032945086; CS-5929; compound 8 [PMID: 26505898]; HY-19322; J3.552.648J; US8592455, 70; 5H7; 2-Pyr
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 440.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
