Details of the Drug
General Information of Drug (ID: DMU6JOK)
| Drug Name |
cirazoline
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
cirazoline; Cirazoline [INN]; Cirazolinum [INN-Latin]; Cirazolina [INN-Spanish]; UNII-QK318GVY3Y; 59939-16-1; LD-3098; QK318GVY3Y; CHEMBL13852; NCGC00015196-04; 2-((o-Cyclopropylphenoxy)methyl)-2-imidazoline; DSSTox_CID_25131; DSSTox_RID_80692; DSSTox_GSID_45131; Cirazolina; Cirazolinum; 1H-Imidazole, 2-((2-cyclopropylphenoxy)methyl)-4,5-dihydro-; 2-[(2-cyclopropylphenoxy)methyl]-4,5-dihydro-1H-imidazole; CAS-59939-16-1; LD 3098; Tocris-0888; Lopac-C-223; AC1L1EEZ; Lopac0_000354; KBioGR_000306; BSPBio_001586; KBioSS_000306; GTPL515
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
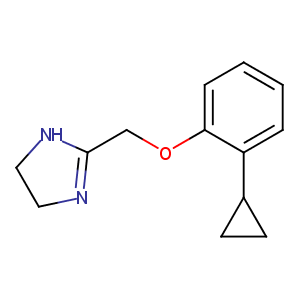 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 216.28 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
