Details of the Drug
General Information of Drug (ID: DMU9BCD)
| Drug Name |
NS-018
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NS-018; ilginatinib; 1239358-86-1; UNII-56R994WX4L; 56R994WX4L; GTPL7839; SCHEMBL14954406; UQTPDWDAYHAZNT-AWEZNQCLSA-N; NS018; ZINC68245917; HY-19631A; AKOS030526348; DB12784; CS-5358; SB16921; 2,6-Pyridinediamine, N2-((1S)-1-(4-fluorophenyl)ethyl)-4-(1-methyl-1H-pyrazol-4-yl)-N6-2-pyrazinyl-; (S)-N2-[1-(4-fluorophenyl)ethyl]-4-(1-methyl-1H-pyrazol-4-yl)-N6-(pyrazin-2yl)pyridine-2,6-diamine; (S)-N-(1-(4-Fluorophenyl)ethyl)-4-(1-methyl-1H-pyrazol-4-yl)-N'-(pyrazin-2-yl)pyridine-2,6-diamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
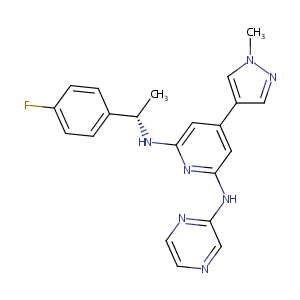 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 389.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
