Details of the Drug
General Information of Drug (ID: DMUF0BJ)
| Drug Name |
CYTISINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CYTISINE; 485-35-8; Sophorine; Baptitoxine; (-)-Cytisine; Laburnin; Baptitoxin; Tabex; Ulexin; Cytiton; Ulexine; Tsitizin; Cytitone; Cystisine; Cytizin; Citizin; Tabax; (1R,5S)-3,4,5,6-Tetrahydro-1H-1,5-methanopyrido[1,2-a][1,5]diazocin-8(2H)-one; Baphitoxine; UNII-53S5U404NU; Cytisine (-); HSDB 3560; EINECS 207-616-0; NSC 407282; BRN 0083882; Cytisin; (1r,5s)-1,2,3,4,5,6-Hexahydro-8h-1,5-Methanopyrido[1,2-A][1,5]diazocin-8-One; CHEBI:4055; CHEMBL497939; 53S5U404NU; Cytisine, 98%
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
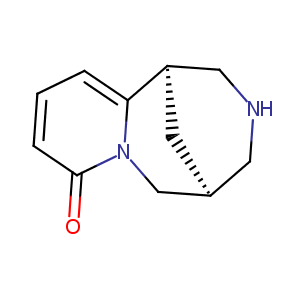 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 190.24 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Tobacco dependence | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6C4A.2 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
References
