Details of the Drug
General Information of Drug (ID: DMUOFM4)
| Drug Name |
Sernivo
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Diprosone; Diprolene; Diprolene AF; Maxivate; Diproderm; Diprosis; Alphatrex; Psorion; 5593-20-4; Rinderon DP; Betamethasone-17,21-dipropionate; Betamethasone 17,21-dipropionate; Sch-11460; Sch 11460; UNII-826Y60901U; beta-Methasone 17,21-dipropionate; EINECS 227-005-2; S-3440; BRN 3638108; CHEBI:31276; CIWBQSYVNNPZIQ-XYWKZLDCSA-N; S 3440; 826Y60901U; 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate; 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-dien
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
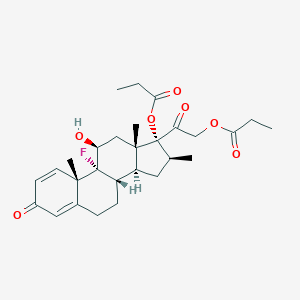 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
