Details of the Drug
General Information of Drug (ID: DMUPN01)
| Drug Name |
AR-A014418
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
487021-52-3; GSK-3beta Inhibitor VIII; 1-(4-methoxybenzyl)-3-(5-nitrothiazol-2-yl)urea; 1-[(4-methoxyphenyl)methyl]-3-(5-nitro-1,3-thiazol-2-yl)urea; A Inhibitor VIII; N-(4-METHOXYBENZYL)-N'-(5-NITRO-1,3-THIAZOL-2-YL)UREA; UNII-87KSH90Q6D; AR-AO 14418; SN 4521; AR-A 014418; CHEMBL259850; 87KSH90Q6D; N-[(4-Methoxyphenyl)methyl]-N'-(5-nitro-2-thiazolyl)urea; AK175829; C12H12N4O4S; N-(4-Methoxybenzyl)-N& -(5-nitro-1,3-thiazol-2-yl)urea; AR 014418; GSK 3be
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
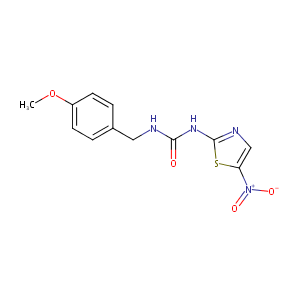 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 308.32 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
