Details of the Drug
General Information of Drug (ID: DMV1408)
| Drug Name |
SU5403
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
189894-57-3; 1-((1S,2S)-1-Hydroxy-1-(4-hydroxyphenyl)propan-2-yl)-4-phenylpiperidin-4-ol methanesulfonate trihydrate; UNII-BD2A56I30W; BD2A56I30W; Traxoprodil mesylate, CP-101606-27; Traxoprodil mesylate [USAN:INN]; (1S,2S)-1-(4-HYDROXYPHENYL)-2-(4-HYDROXY-4-PHENYLPIPERIDIN-1-YL)-1-PROPANOL METHANESULFONATE TRIHYDRATE; Traxoprodil mesylate hydrate; CHEMBL3989673; KS-00000REE; MolPort-023-219-113; AKOS025396623; RL02411; DS-5988; BCP9000543
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
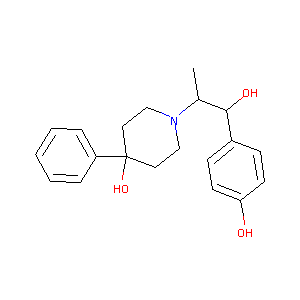 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight | 477.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 7 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 10 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
