Details of the Drug
General Information of Drug (ID: DMVTLF9)
| Drug Name |
(10r)-10-Formyl-5,8,10-Trideazafolic Acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(10R)-10-FORMYL-5,8,10-TRIDEAZAFOLIC ACID; NHR; 2-{4-[2-(2-AMINO-4-HYDROXY-QUINAZOLIN-6-YL)-1-CARBOXY-ETHYL]-BENZOYLAMINO}-PENTANEDIOIC ACID; 1c3e; AC1L9GSR; DB04264; 8937-EP2308866A1; 8937-EP2308846A2; 8937-EP2305697A2; 8937-EP2298780A1; 8937-EP2277872A1; 8937-EP2316829A1; 8937-EP2308872A1; 8937-EP2308845A2; 8937-EP2305698A2; 8937-EP2305695A2; 8937-EP2301932A1; 8937-EP2287167A1; 8937-EP2283898A1; 8937-EP2281563A1; 8937-EP2316459A1; 8937-EP2308844A2; 8937-EP2277869A1; 8937-EP2270018A1; 8937-EP2308812A2; 8937-EP2305696A2
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
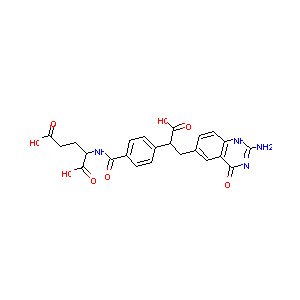 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 482.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
