Details of the Drug
General Information of Drug (ID: DMWC70I)
| Drug Name |
N-(2,3,4,9-Tetrahydro-1H-carbazol-3-yl)-acetamide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
60480-69-5; N-(2,3,4,9-TETRAHYDRO-1H-CARBAZOL-3-YL)ACETAMIDE; CHEMBL41973; 3-Acetamido-1,2,3,4-tetrahydrocarbazole; N-(2,3,4,9-Tetrahydro-1H-carbazol-3-yl)-acetamide; SCHEMBL7406266; KS-00000TMN; CTK2F0321; DTXSID90494196; MolPort-042-663-532; DNJBLXBGYRYOBT-UHFFFAOYSA-N; BDBM50212923; AKOS027461084; 3-Acetamido-1,2,3,4-tetrahydrocarbazol; DS-19200; AK543106; Acetamide, N-(2,3,4,9-tetrahydro-1H-carbazol-3-yl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
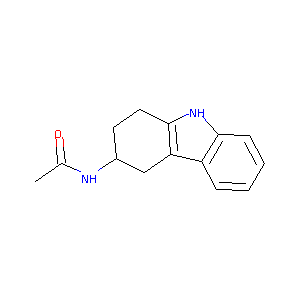 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 228.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
