Details of the Drug
General Information of Drug (ID: DMWCJEU)
| Drug Name |
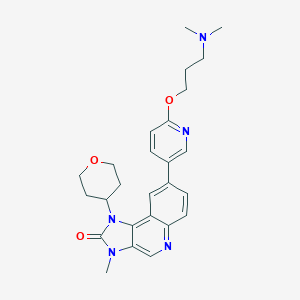
AZD0156
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AOTRIQLYUAFVSC-UHFFFAOYSA-N; 1821428-35-6; AZD-0156; UNII-P5T0XWC07Z; P5T0XWC07Z; GTPL9942; CHEMBL3960662; SCHEMBL17246146; AZD 0156 [WHO-DD]; MolPort-044-560-374; BDBM245474; BCP18990; EX-A1321; s8375; ZINC498035578; AKOS030629510; CS-5889; SB19769; AZD0156 (AZD-0156); AS-35329; compound 64 [PMID: 29683659]; HY-100016; US9428503, 1; 8-[6-[3-(dimethylamino)propoxy]pyridin-3-yl]-3-methyl-1-(oxan-4-yl)imidazo[4,5-c]quinolin-2-one; 8-(6-(3-(dimethylamino)propoxy)pyridin-3-yl)-3-methyl-1-(tetrahydro-2H-pyran-4-yl)-1H-imidazo[
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 461.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
