Details of the Drug
General Information of Drug (ID: DMWCTZ7)
| Drug Name |
decynium 22
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pseudoisocyanine; 1,1'-Diethyl-2,2'-cyanine; 1-Ethyl-2-((1-ethyl-2(1H)-quinolylidene)methyl)quinolinium; 20766-49-8; CHEMBL1197556; CHEBI:37994; Pseudocyanine; 1-ethyl-2-[(E)-(1-ethylquinolin-2(1H)-ylidene)methyl]quinolinium; Diethylpseudoisocyanine; MLS000532635; AC1NUNMU; AC1Q1IYW; SMR000137574; AC1L2OT9; CBDivE_000769; SCHEMBL737002; 977-96-8 (iodide); 2402-42-8 (chloride); SCHEMBL13619260; 1613-31-6 (bromide); BDBM39687; cid_5717105; CHEBI:38002; DTXSID40174835; MolPort-006-385-585; STK396423; (E)-1,1'-diethyl-2,2'-cyanine; Dy22; 1,1'-diethyl-2,2'-cyanine; NSC-97374
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
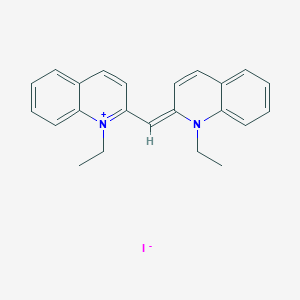 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 454.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
