Details of the Drug
General Information of Drug (ID: DMWK3RO)
| Drug Name |
(1-Benzyl-2-oxo-ethyl)-carbamic acid benzyl ester
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cbz-L-Phenylalaninal; 59830-60-3; (S)-Benzyl (1-oxo-3-phenylpropan-2-yl)carbamate; CHEMBL412423; AK-43912; N-Cbz-phenylalaninal; PubChem16008; N-Cbz-L-Phenylalaninal; AC1ODUQ7; (1-Benzyl-2-oxo-ethyl)-carbamic acid benzyl ester; SCHEMBL630290; KS-00000FVM; DTXSID40427338; ZX-AFC000446; MolPort-009-682-951; HZDPJHOWPIVWMR-INIZCTEOSA-N; n-benzyloxycarbonyl-l-phenylalaninal; ZINC2569810; BDBM50014568; 8910AB; ANW-72305; AKOS005146049; CC-1855; KB-75975; N-((Benzyloxy)carbonyl)-L-phenylalaninal; AC-23793; DS-14328; AJ-41774; AX8120205
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
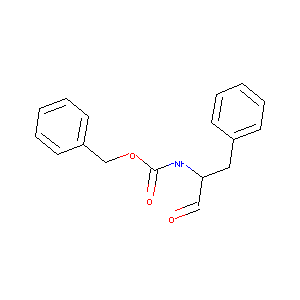 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 283.32 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
