Details of the Drug
General Information of Drug (ID: DMWSLTB)
| Drug Name |
5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-ol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
9-Hydroxyellipticine; 9-Hydroxyellipticin; 51131-85-2; 5,11-dimethyl-6H-pyrido[4,3-b]carbazol-9-ol; Hydroxyellipticine; ELLIPTICINE, 9-HYDROXY-; UNII-9G4A3ET6XG; IGIG 929; Hydroxy-9 ellipticine [French]; EINECS 257-000-0; NSC 237070; NSC 210717; 9G4A3ET6XG; CHEMBL26559; CHEBI:88297; C17H14N2O; 5,11-Dimethyl-6H-pyrido(4,3-b)carbazol-9-ol; 6H-Pyrido(4,3-b)carbazol-9-ol, 5,11-dimethyl-; 6H-Pyrido[4,3-b]carbazol-9-ol, 5,11-dimethyl-; 9-hydroxy-5,11-dimethyl-6H-pyrido[4,3-b]carbazole; Hydroxy-9 ellipticine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
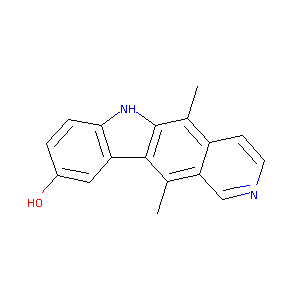 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 262.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
