Details of the Drug
General Information of Drug (ID: DMWUG4L)
| Drug Name |
PRAX-562
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Relutrigine; PRAX-562; 2392951-29-8; 3-[Ethoxy(difluoro)methyl]-6-[5-fluoro-6-(2,2,2-trifluoroethoxy)pyridin-3-yl]-[1,2,4]triazolo[4,3-a]pyrazine; relutrigine [INN]; AW5C5XHM4W; PRAX562; SCHEMBL23132181; GTPL12643; BFXBSYMVTNEFRF-UHFFFAOYSA-N; EX-A7203; HY-148792; CS-0641132; 1,2,4-TRIAZOLO(4,3-A)PYRAZINE, 3-(ETHOXYDIFLUOROMETHYL)-6-(5-FLUORO-6-(2,2,2-TRIFLUOROETHOXY)-3-PYRIDINYL)-; 3-(ethoxydifluoromethyl)-6-[5-fluoro-6-(2,2,2-trifluoroethoxy)pyridin-3- yl][1,2,4]triazolo[4,3-a]pyrazine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
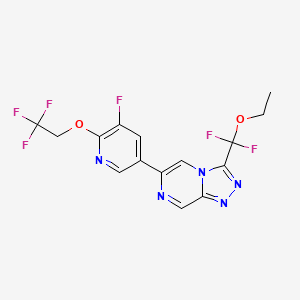 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
References
