Details of the Drug
General Information of Drug (ID: DMWZ2TU)
| Drug Name |
CO-1686
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1374640-70-6; AVL-301; CO1686; UNII-72AH61702G; CNX-419; CO-1686 (AVL-301); Rociletinib(AVL-301,CNX-419,CO-1686); 72AH61702G; N-(3-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-(trifluoromethyl)pyrimidin-4-yl)amino)phenyl)acrylamide; CO 1686; Rociletinib (CO-1686, AVL-301); Rociletinib [USAN:INN]; Tube721; Rociletinib (USAN/INN); Rociletinib (CO-1686); SCHEMBL4177736; GTPL7966; CHEMBL3545308; EX-A228; MolPort-035-395-816; C27H28F3N7O3; HMS3653G08; BDBM149404; BCP07085; AOB87314; ZINC98043800; s7284
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
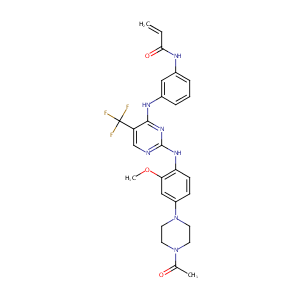 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 555.6 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Lung cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C25.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
