Details of the Drug
General Information of Drug (ID: DMX0C5Z)
| Drug Name |
APL-130277
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Apomorphine hydrochloride hemihydrate; UNII-F39049Y068; Apmorphine hydrochloride hemihydrate; Apomorphin hydrochlorid wasser (2/1); Apomorphine hydrochloride [USP]; 6abeta-Aporphine-10,11-diol hydrochloride hemihydrate; Apomorphine hydrochloride hydrate; F39049Y068; Uprima (TN); DSSTox_RID_82721; DSSTox_CID_28159; DSSTox_GSID_48185; Apomorphine hydrochloride (USP); 58-00-4 (Parent); CHEBI:31228; 4H-Dibenzo(de,g)quinoline-10,11-diol, 5,6,6a,7-tetrahydro-6-methyl-, hydrochloride, hydrate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
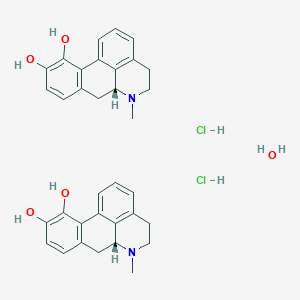 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
