Details of the Drug
General Information of Drug (ID: DMX1KDM)
| Drug Name |
ABI-H0731
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Vebicorvir; UNII-16F6055SMG; 16F6055SMG; 11-Oxo-N-((2-(trifluoromethyl)thiazol-5-yl)methyl)-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8-carboxamide 5,5-dioxide; 2090064-66-5; Vebicorvir [INN]; Vebicorvir [USAN]; SCHEMBL21250344; WHO 11201; Dibenzo(b,F)(1,4)thiazepine-8-carboxamide, 10,11-dihydro-11-oxo-N-((2-(trifluoromethyl)-5-thiazolyl)methyl)-, 5,5-dioxide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
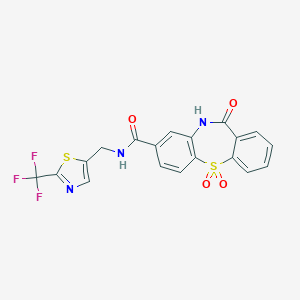 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 467.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
