Details of the Drug
General Information of Drug (ID: DMX4DUR)
| Drug Name |
2-(3-bromobenzoylamino)-4-chlorobenzoic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
827325-60-0; 2-[(3-bromobenzoyl)amino]-4-chlorobenzoic acid; CHEMBL225612; 2-(3-Bromobenzamido)-4-chlorobenzoic acid; Benzoic acid, 2-[(3-bromobenzoyl)amino]-4-chloro-; 2-(3-bromobenzoylamino)-4-chlorobenzoic acid; AC1N8QX1; SCHEMBL14075271; CTK3D6918; KS-00003MRF; DTXSID50401927; MolPort-002-885-931; ZINC4104976; ZX-AN021268; ALBB-022752; BDBM50158557; AKOS005107642; MCULE-7998531013; MS-0095; R4909; SR-01000309735; SR-01000309735-1; 2-[(3-bromobenzoyl)amino]-4-chlorobenzenecarboxylic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
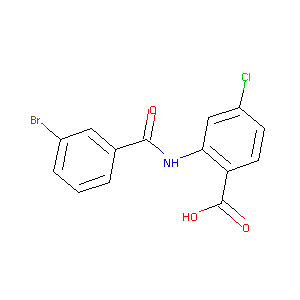 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 354.58 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
