Details of the Drug
General Information of Drug (ID: DMXC9VY)
| Drug Name |
PF-07054894
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PF-07054894; SCHEMBL21803033; GTPL12765; EX-A7899; HY-148531; CS-0634382; PF07054894; 2413693-96-4; 4-[[2-[[(R)-(1,4-dimethylpyrazol-3-yl)-(1-methylcyclopentyl)methyl]amino]-3,4-dioxocyclobuten-1-yl]amino]-3-hydroxy-N,N-dimethylpyridine-2-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
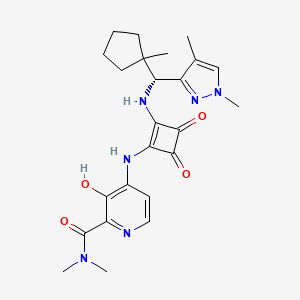 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
