Details of the Drug
General Information of Drug (ID: DMXFLMK)
| Drug Name |
4-(3-cyclohexylureido)butanoic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-{[(Cyclohexylamino)carbonyl]amino}butanoic acid; 4-{[(CYCLOHEXYLAMINO)CARBONYL]AMINO}BUTANOIC ACID; 4-[(cyclohexylcarbamoyl)amino]butanoic acid; 511552-46-8; 4-(3-Cyclohexyl-ureido)-butyric acid; CHEMBL219695; 4-([(Cyclohexylamino)carbonyl]amino)butanoic acid; 4-(3-CYCLOHEXYLURIEDO)-BUTYRIC ACID; NC4; 1zd3; AC1O4WJY; AC1Q75I7; SCHEMBL1065092; WSVFRGGLURJIMG-UHFFFAOYSA-N; MolPort-004-290-136; HMS3604L06; ZINC8754389; BDBM50192936; AKOS000125413; 4-(3-cyclohexylur-eido)-butyric acid; NE13821; MCULE-1918218076; DB08257
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
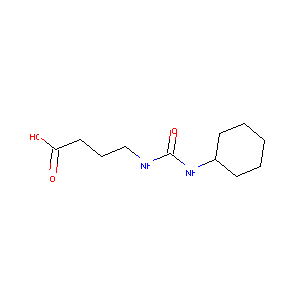 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 228.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
