Details of the Drug
General Information of Drug (ID: DMXFZZB)
| Drug Name |
Tofogliflozin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TOFOGLIFLOZIN; 903565-83-3; CSG 452; Tofogliflozin anhydrous; UNII-554245W62T; CSG-452; 554245W62T; deberza; apleway; Tofogliflozin [INN]; R-7201; Tofogliflozin anyhydrous; Tube107; EC 619-989-0; SCHEMBL903156; GTPL9395; CHEMBL2110731; DTXSID90238097; CSG452;CSG-452; CHEBI:136041; MolPort-044-723-920; ZINC35826342; BDBM50396779; AKOS027250822; DB11824; SB11170; compound 16d [PMID: 22889351]; NCGC00485920-01; KB-76204
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
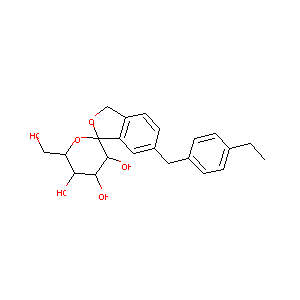 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
