Details of the Drug
General Information of Drug (ID: DMXI9BP)
| Drug Name |
Brilacidin
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-I1679X069H; 1224095-98-0; I1679X069H; Brilacidin [USAN:INN]; Brilacidin (USAN); SCHEMBL2878543; CHEMBL2219413; DTXSID90153594; AKOS030238222; DB12997; CS-6677; HY-19892; D10414; N,N'-Bis(3-((5-(carbamimidoylamino)pentanoyl)amino)-2-((3R)-pyrrolidin-3-yloxy)-5-(trifluoromethyl)phenyl)pyrimidine-4,6-dicarboxamide; 4,6-Pyrimidinedicarboxamide, N4,N6-bis(3-((5-((aminoiminomethyl)amino)-1-oxopentyl)amino)-2-((3R)-3-pyrrolidinyloxy)-5-(trifluoromethyl)phenyl)-
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
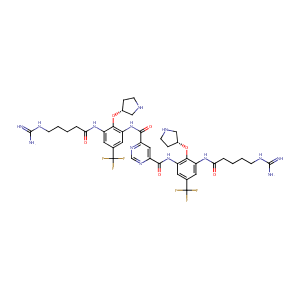 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 936.9 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 20 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 10 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 18 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
