Details of the Drug
General Information of Drug (ID: DMXRK1D)
| Drug Name |
Tafamidis meglumine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tafamidis meglumine; Fx-1006A; Fx1006A; Fx 1006A; UNII-ZU7CF08A1A; ZU7CF08A1A; 951395-08-7; CHEBI:79345; Tafamidis meglumine [USAN:INN]; SCHEMBL14783506; CHEMBL2105675; Tafamidis meglumine (JAN/USAN); SB16821; D-Glucitol, 1-deoxy-1-(methylamino)-, 2-(3,5-dichlorophenyl)-6-benzoxazolecarboxylate; FT-0700926; D09674; 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid--1-deoxy-1-(methylamino)-D-glucitol (1/1); 1-deoxy-1-(methylazaniumyl)-D-glucitol 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylate
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
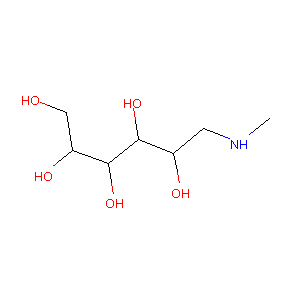 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
