Details of the Drug
General Information of Drug (ID: DMXUH6W)
| Drug Name |
JTV-803
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-7PT6AGF1ST; JTV 803; 7PT6AGF1ST; 247131-79-9; JTV-803; SCHEMBL6191062; NOBZETMXGVAWIM-UHFFFAOYSA-N; 4-Piperidinecarboxylic acid, 4-(((2-(aminoiminomethyl)-1,2,3,4-tetrahydro-7-isoquinolinyl)oxy)methyl)-1-(4-pyridinyl)-, monomethanesulfonate; 4-Piperidinecarboxylic acid, 4-(((2-(aminoiminomethyl)-1,2,3,4-tetrahydro-7-isoquinolinyl)oxy)methyl)-1-(4-pyridinyl)-, methanesulfonate (1:1); KB-78007; 4-(2-amidino-1,2,3,4-tetrahydroisoquinolin-7-yloxymethyl)-1-(pyridin-4-yl)piperidine-4-carboxylic acid methanesulfonate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
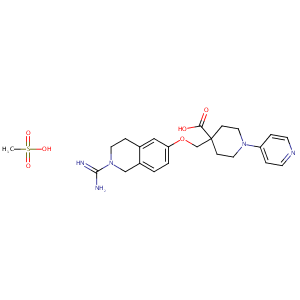 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight | 505.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Thrombosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DB61-GB90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
