Details of the Drug
General Information of Drug (ID: DMXWYMH)
| Drug Name |
GNF-PF-4292
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Hydrocinchonine; GNF-PF-4292; CHEMBL428695; Cinchotine; (8alpha,9R)-10,11-Dihydrocinchonan-9-ol; 485-64-3; Cinchonifine; Pseudocinchonine; y-Cinchonine; 10,11-dihydrocinchonan-9-ol; (9S)-10,11-Dihydrocinchonan-9-ol; 10,11-dihydrocinchonine; AC1Q4XW7; AC1L2RE5; Oprea1_425428; SCHEMBL312806; MolPort-003-871-861; WFJNHVWTKZUUTR-UHFFFAOYSA-N; BDBM50047001; NCGC00142479-01; AC-30196; ST056343; FT-0700733; (9S)-10,11-Dihydrocinchonan-9-ol, 9CI; (1R)(5-ethylquinuclidin-2-yl)-4-quinolylmethan-1-ol; Quinoline 4-(alpha-(7-ethyl-1,4-ethanopip
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
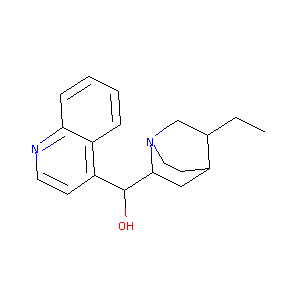 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 296.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
