Details of the Drug
General Information of Drug (ID: DMXYDMP)
| Drug Name |
LM10
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1316695-35-8; (E)-3-(2-(1H-TETRAZOL-5-YL)VINYL)-6-FLUORO-1H-INDOLE; CHEMBL1812545; LM-10; 6-fluoro-3-[(E)-2-(1H-1,2,3,4-tetrazol-5-yl)ethenyl]-1H-indole; 6-Fluoro-3-[(1E)-2-(2H-tetrazol-5-yl)ethenyl]-1H-indole; 1H-Indole, 6-fluoro-3-[(1E)-2-(2H-tetrazol-5-yl)ethenyl]-; LM 10; GTPL9016; SCHEMBL16820602; SCHEMBL16820603; BDBM311862; CS-D0342; BDBM50350248; MFCD26097257; s8368; US10155972, Compound LM10; ZINC72108665; AKOS024464444; CCG-266788; AS-30527; Q27081058
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
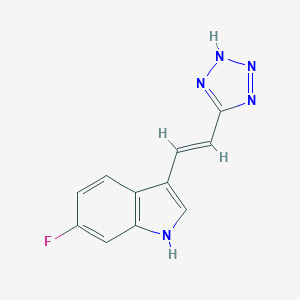 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 229.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
