Details of the Drug
General Information of Drug (ID: DMY8O0B)
| Drug Name |
CP-320626
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CP-320626; UNII-FKX709RK3Q; FKX709RK3Q; CHEMBL99889; 5-CHLORO-1H-INDOLE-2-CARBOXYLIC ACID [1-(4-FLUOROBENZYL)-2-(4-HYDROXYPIPERIDIN-1YL)-2-OXOETHYL]AMIDE; 5-chloro-N-[(2S)-3-(4-fluorophenyl)-1-(4-hydroxypiperidin-1-yl)-1-oxopropan-2-yl]-1H-indole-2-carboxamide; YDCGVASFVACWKF-NRFANRHFSA-N; AC1L9GTI; 1H-Indole-2-carboxamide, 5-chloro-N-((1S)-1-((4-fluorophenyl)methyl)-2-(4-hydroxy-1-piperidinyl)-2-oxoethyl)-; 186430-23-9; SCHEMBL7234251; BDBM35346; DB03383; 1H-Indole-2-carboxamide, 5-chloro-N-(1-((4-fluorophenyl)methyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
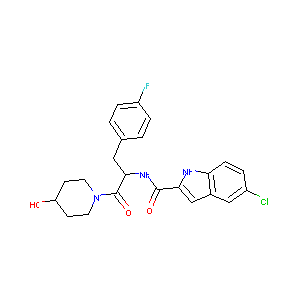 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 443.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
