Details of the Drug
General Information of Drug (ID: DMYFNW9)
| Drug Name |
JATRORRHIZINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Jatrorrhizine; 3621-38-3; neprotin; jateorrhizine; Yatrorizine; UNII-091S1F8V5Q; CHEMBL251055; CHEBI:6087; GNF-PF-220; 7,8,13,13a-tetradehydro-3-hydroxy-2,9,10-trimethoxyberbinium; 091S1F8V5Q; 2,9,10-trimethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium-3-ol; Dibenzo[a,g]quinolizinium, 5,6-dihydro-3-hydroxy-2,9,10-trimethoxy-; 3-Hydroxy-2,9,10-trimethoxy-5,6-dihydroisoquinolino[3,2-a]isoquinolin-7-ium; 3-Hydroxy-2,9,10-trimethoxy-5,6-dihydroisoquinolino-[3,2-a]isoquinolin-7-ium; NSC645313; NSC150445; NSC209410
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
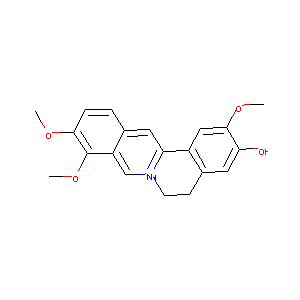 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 338.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
