Details of the Drug
General Information of Drug (ID: DMYILGK)
| Drug Name |
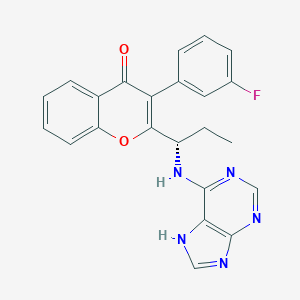
RP6530
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
tenalisib; HDXDQPRPFRKGKZ-INIZCTEOSA-N; Tenalisib; 1639417-53-0; UNII-2261HH611H; 2261HH611H; Tenalisib [INN]; GTPL9907; SCHEMBL16279460; AKOS027325582; RP-6530; RP 6530; CS-6375; HY-17645; 4H-1-Benzopyran-4-one, 3-(3-fluorophenyl)-2-((1S)-1-(9H-purin-6-ylamino)propyl)-; (S)-2-(1-((7H-purin-6-yl)amino)propyl)-3-(3-fluorophenyl)-4H-chromen-4-one; 3-(3-fluorophenyl)-2-[(1S)-1-[(9H-purin-6-yl)amino]propyl]-4H-chromen-4-one; 3-(3-Fluorophenyl)-2-((1S)-1-((7H-purin-6-yl)amino)propyl)-4H-1-benzopyran-4-one; 1693773-94-2
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 415.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic lymphocytic leukaemia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A82.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
