Details of the Drug
General Information of Drug (ID: DMYN391)
| Drug Name |
Odevixibat
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
501692-44-0; UNII-2W150K0UUC; 2W150K0UUC; A4250; AZD8294; 501692-44-0 (free base); Odevixibat (USAN); Odevixibat [USAN]; AR-H064974; A-4250; (S)-2-((R)-2-(2-((3,3-Dibutyl-7-(methylthio)-1,1-dioxido-5-phenyl-2,3,4,5-tetrahydrobenzo[f][1,2,5]thiadiazepin-8-yl)oxy)acetamido)-2-(4-hydroxyphenyl)acetamido)butanoic acid; SCHEMBL946468; CHEMBL4297588; BDBM77040; GTPL11194; AZD-8294; example 5 [US9694018B1]; WHO 10706; HY-109120; Unk-D-nTyr-Abu-OH (IUPAC condensed name); CS-0078340; D11716; US9694018, 5; (2S)-2-(((2R)-2-((((3,3-Dibutyl-7-(methylthio)-1,1-dioxido-5-phenyl-2,3,4,5-tetrahydro- 1,2,5-benzothiadiazepin-8-yl)oxy)acetyl)amino)-2-(4-hydroxyphenyl)acetyl)amino)butanoic acid; (2S)-2-[[(2R)-2-[[2-[(3,3-dibutyl-7-methylsulfanyl-1,1-dioxo-5-phenyl-2,4-dihydro-1lambda6,2,5-benzothiadiazepin-8-yl)oxy]acetyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]butanoic acid; Butanoic acid, 2-(((2R)-((((3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,2,5-benzothiadiazepin-8-yl)oxy)acetyl)amino)(4-hydroxyphenyl)acetyl)amino)-, (2S)-; Butanoic acid, 2-(((2R)-2-((2-((3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,2,5-benzothiadiazepin-8-yl)oxy)acetyl)amino)-2-(4-hydroxyphenyl)acetyl)amino)-, (2S)-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
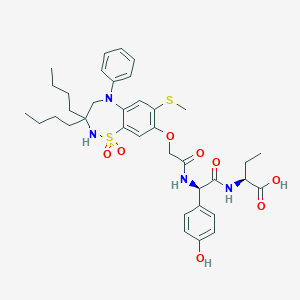 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 740.9 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 17 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pruritus | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EC90 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
