Details of the Drug
General Information of Drug (ID: DMYR1K8)
| Drug Name |
S-17092-1
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NXSXRIHXEQSYEZ-KNJMJIDISA-N; S 17092; CHEMBL1086968; 176797-26-5; S-17092; s17092; SCHEMBL194654; GTPL6565; DTXSID70433003; ZINC3825778; BDBM50316818; NCGC00485216-01; S 17092, > ((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbonyl)octahydro-1H-indol-2-yl)(thiazolidin-3-yl)methanone; (2S,3aS,7aS)-1-(((R,R)-2-Phenylcyclopropyl)carbonyl)-2-((thiazolidin-3-yl)carbonyl)octahydro-1H-indole; [(2S,3aS,7aS)-Octahydro-1-[[(1R,2R)-2-phenylcyclopropyl]carbonyl]-1H-indol-2-yl]-3-thiazolidinyl--methanone; S-17092 (undefined isomer); (2S,3aS,7aS)-1-[(1R,2R)-2-Phenylcyclopropylcarbonyl]-2-(thiazolidin-3-ylcarbonyl)perhydroindole
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
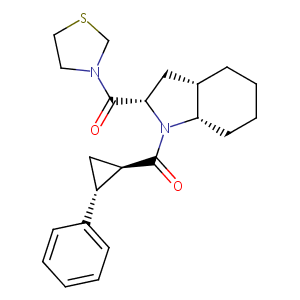 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 384.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Cognitive impairment | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6D71 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
