Details of the Drug
General Information of Drug (ID: DMYR5OX)
| Drug Name |
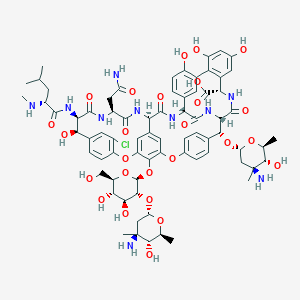
EREMOMYCIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Hexadecino[4,5-m][10,2,16]benzoxadiazacyclotetracosine-26-carboxylic acid; (4"R)-22-O-(3-Amino-2,3,6-trideoxy-3-C-methyl-alpha-L-arabino-hexopyranosyl)-19-dechlorovancomycin; Etrahydroxy-6-(N2-methylleucylamido)-2,5,24,38,39-pentaoxo-2,3,4,5,6,7,23,24,25,26,36,37,38,38a-tetradecahydro-1H,22H-8,11:18,21-dietheno-23,36-(iminomethano)-13,16:31,35-dimetheno[1,6,9]oxadiazacyclo; 22-(3-Amino-2,3,6-trideoxy-3-C-methyl-L-arabinohexopyranosyloxy)-44-[2-O-(3-amino-2,3,6-trideoxy-3-C-methyl-L-arabinohexopyranosyl)-beta-D-glucopyranosyloxy]-3-(carbamoylmethyl)-10-chloro-7,28,30,32-t; 3-(Carbamoylmethyl)-10-chloro-7,28,30,32-tetrahydroxy-6-(N2-methylleucylamido)-2,5,24,38,39-pentaoxo-22-(4-epi-vancosaminyloxy)-44-[2-O-(4-epi-vancosaminyl)-beta-D-glucopyranosyloxy]-2,3,4,5,6,7,23,24; 35-dimetheno[1,6,9]oxadiazacyclohexadecino[4,5-m][10,2,16]benzoxadiazacyclotetracosine-26-carboxylic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1558 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 15 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 20 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 29 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
