Details of the Drug
General Information of Drug (ID: DMZMFX6)
| Drug Name |
KU-1257
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dalcotidine; 120958-90-9; UNII-9968S2UKFJ; KU-1257; KU 1257; 9968S2UKFJ; NCGC00182982-01; Dalcotidine [INN]; Dalcotidine (JAN/INN); AC1L2VE9; DSSTox_RID_83001; DSSTox_CID_28732; DSSTox_GSID_48806; CHEMBL311206; SCHEMBL1815082; N-Ethyl-N'-(3-(3-(piperidinomethyl)phenoxy)propyl)urea; DTXSID4048806; CHEBI:31454; 1-ethyl-3-[3-[3-(piperidin-1-ylmethyl)phenoxy]propyl]urea; ZINC1889603; Tox21_113283; BDBM50406670; CAS-120958-90-9; D01698; L002431; A1-04840; 1-Ethyl-3-(3-((alpha-piperidino-m-tolyl)oxy)propyl)urea; Urea, N-ethyl-N'-(3-(3-(1-p
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
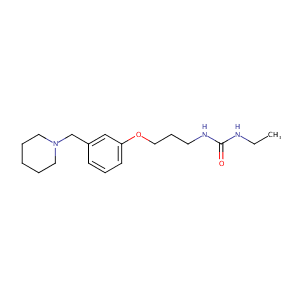 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 319.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
