Details of the Drug
General Information of Drug (ID: DMZR02J)
| Drug Name |
Benzo[c][1,2]oxaborol-1(3H)-ol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5735-41-1; 1-Hydroxy-2,1-benzoxaborolane; benzo[c][1,2]oxaborol-1(3H)-ol; 2,1-benzoxaborol-1(3h)-ol; monoester; 1,3-dihydro-1-hydroxy-2,1-benzoxaborole; 1,3-DIHYDRO-2,1-BENZOXABOROL-1-OL; 2,1-Benzoxaborole, 1,3-dihydro-1-hydroxy-; CHEBI:78238; 1-hydroxy-3H-2,1-benzoxaborole; 2-(Hydroxymethyl)phenylboronic Acid Cyclic Monoester; 2-(HYDROXYMETHYL)PHENYLBORONIC ACID, DEHYDRATE; KSC-6-288; NSC719278; PubChem5079; 2,1-benzoxaborol-1-ol; 2-Oxa-1-boraindan-1-ol; AMTB152; C7H7BO2; 3H-2,1-benzoxaborol-1-ol; 1-hydroxy-2,1-benzoxaborole
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
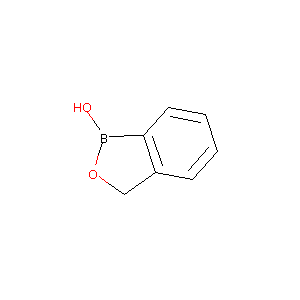 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 133.94 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
