| 1 |
ClinicalTrials.gov (NCT01215851) Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis With(J-M-Pa-Z) (NC-001)
|
| 2 |
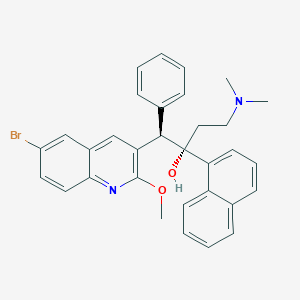
Bedaquiline FDA Label
|
| 3 |
Nat Rev Drug Discov. 2013 Feb;12(2):87-90.
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019
|
| 5 |
ClinicalTrials.gov (NCT02342886) Shortening Treatment by Advancing Novel Drugs. U.S. National Institutes of Health.
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 7 |
Bedaquiline metabolism: enzymes and novel metabolites. Drug Metab Dispos. 2014 May;42(5):863-6.
|
| 8 |
FDA briefing document - pretomanid tablet, 200 mg meeting of the antimicrobial drugs advisory committee (AMDAC).
|
| 9 |
ClinicalTrials.gov (NCT01691534) Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis With Clofazimine (C)-TMC207 (J)-PA-824 (Pa)-Pyrazinamide (Z). U.S. National Institutes of Health.
|
|
|
|
|
|
|