| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
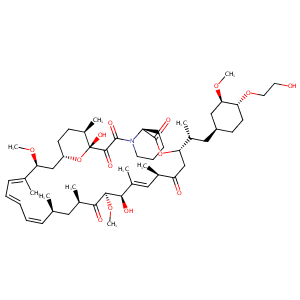
Everolimus FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
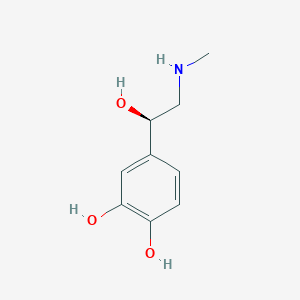
Epinephrine FDA Label
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 509).
|
| 8 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 9 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 10 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 11 |
Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: involvement of beta1- and beta2-adrenergic receptors. Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G269-77.
|
| 12 |
Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006 Jun;50(8):941-52.
|
| 13 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 14 |
Steroid glucuronides: human circulatory levels and formation by LNCaP cells. J Steroid Biochem Mol Biol. 1991;40(4-6):593-8.
|
| 15 |
Crystal structure of human sulfotransferase SULT1A3 in complex with dopamine and 3'-phosphoadenosine 5'-phosphate. Biochem Biophys Res Commun. 2005 Sep 23;335(2):417-23.
|
| 16 |
Adrenal catecholamines and their metabolism in the vitamin A deficient rat. Ann Nutr Metab. 1983;27(3):220-7.
|
| 17 |
Different metabolism of norepinephrine and epinephrine by catechol-O-methyltransferase and monoamine oxidase in rats. J Pharmacol Exp Ther. 1994 Mar;268(3):1242-51.
|
| 18 |
Role of monoamine-oxidase-A-gene variation in the development of glioblastoma in males: a case control study. J Neurooncol. 2019 Nov;145(2):287-294.
|
| 19 |
Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001 Feb;59(2):393-402. doi: 10.1124/mol.59.2.393.
|
| 20 |
Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010 Dec;335(3):743-53. doi: 10.1124/jpet.110.170142. Epub 2010 Sep 21.
|
| 21 |
Epinephrine upregulates superoxide dismutase in human coronary artery endothelial cells. Free Radic Biol Med. 2001 Jan 15;30(2):148-53.
|
| 22 |
Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta. 2003 May 12;1640(2-3):137-42.
|
| 23 |
Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983 Dec 8;309(23):1414-9. doi: 10.1056/NEJM198312083092303.
|
| 24 |
A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest. 1967 Jan;46(1):86-94. doi: 10.1172/JCI105514.
|
| 25 |
Myocardial ischaemia and ventricular arrhthymias precipitated by physiological concentrations of adrenaline in patients with coronary artery disease. Br Heart J. 1992 May;67(5):419-20. doi: 10.1136/hrt.67.5.419-b.
|
| 26 |
Epinephrine facilitates the growth of T cell lymphoma by altering cell proliferation, apoptosis, and glucose metabolism. Chem Biol Interact. 2023 Jan 5;369:110278. doi: 10.1016/j.cbi.2022.110278. Epub 2022 Nov 22.
|
| 27 |
Carvedilol selectively inhibits oscillatory intracellular calcium changes evoked by human alpha1D- and alpha1B-adrenergic receptors. Cardiovasc Res. 2004 Sep 1;63(4):662-72. doi: 10.1016/j.cardiores.2004.05.014.
|
| 28 |
Carvedilol prevents epinephrine-induced apoptosis in human coronary artery endothelial cells: modulation of Fas/Fas ligand and caspase-3 pathway. Cardiovasc Res. 2000 Feb;45(3):788-94. doi: 10.1016/s0008-6363(99)00369-7.
|
| 29 |
The platelet P2Y12 receptor contributes to granule secretion through Ephrin A4 receptor. Platelets. 2012;23(8):617-25. doi: 10.3109/09537104.2011.645924. Epub 2012 Jan 24.
|
| 30 |
Hormone-sensitive lipase in human adipose tissue, isolated adipocytes, and cultured adipocytes. Pediatr Res. 1982 Dec;16(12):982-8. doi: 10.1203/00006450-198212000-00002.
|
| 31 |
Epidermal growth factor facilitates epinephrine inhibition of P2X7-receptor-mediated pore formation and apoptosis: a novel signaling network. Endocrinology. 2005 Jan;146(1):164-74. doi: 10.1210/en.2004-1026. Epub 2004 Sep 30.
|
| 32 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 33 |
Evaluation of cytogenetic and DNA damage in human lymphocytes treated with adrenaline in vitro. Toxicol In Vitro. 2015 Feb;29(1):27-33. doi: 10.1016/j.tiv.2014.08.001. Epub 2014 Aug 27.
|
| 34 |
Enzymatic characterization and interspecies difference of phenol sulfotransferases, ST1A forms. Drug Metab Dispos. 2001 Mar;29(3):274-81.
|
| 35 |
Increased arterial pressure in mice with overexpression of the ADHD candidate gene calcyon in forebrain. PLoS One. 2019 Feb 12;14(2):e0211903. doi: 10.1371/journal.pone.0211903. eCollection 2019.
|
|
|
|
|
|
|